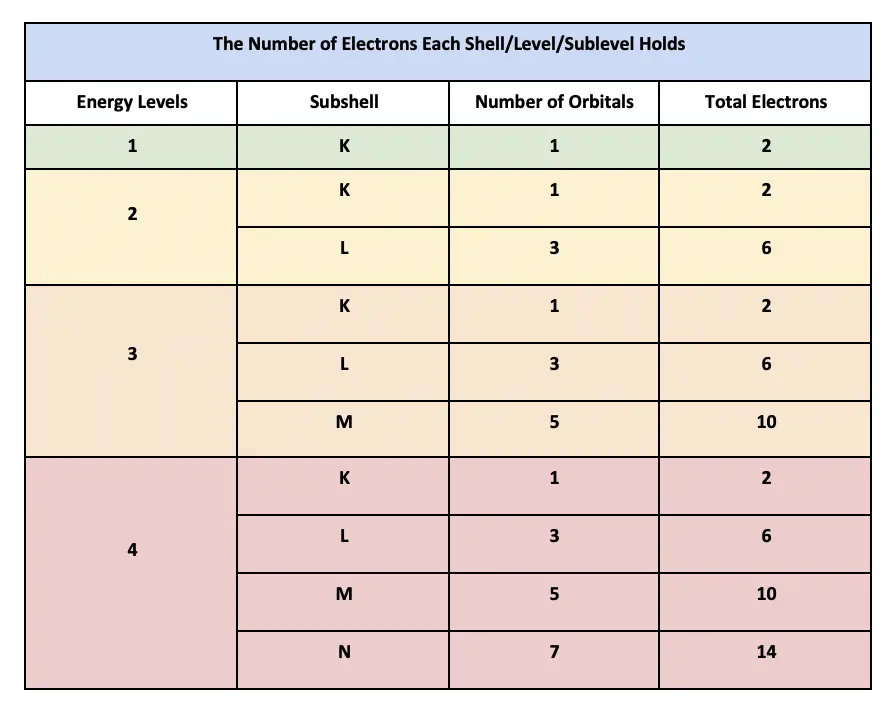
Every electron orbital accommodates no more than two electrons. This makes it possible to assign electrons to a specific electron shell electron subshell and electron orbital.

Orbital Hybridization Chemistry Science Chemistry Nomenclature Chemistry
If ℓ is the angular quantum number of subshell then maximum electrons it can hold is 22ℓ 1 Sub-shellℓMaximum electrons s0220 1 2 p1221 1 6.

. Quantum numbers are used to describe completely the position energy space orientation and possible interaction of electrons in an atom. In class we saw that the area of an ellipse can be determined using a linear transformation and a determinant. Answer -- 2 but how.
2 in the s orbital 6 in the three p orbitals and 10 in the five d orbitals. Neon Ne on the other hand has a total of ten electrons. Two electrons fill the 1s orbital and the third electron then fills the 2s orbital.
Each p orbital holds 6 3 pairs d orbitals hold 10 5 pairs and f orbitals hold 14 7 pairs. Its electron configuration is 1s 2 2s 1. - The Handy Chemistry Answer Book How many electrons can fit in each orbital.
Complete step by step answer. So it holds 2. Nov 212021 - How many electrons can fit in the orbital for which n 3 and l 1.
Electron Distribution Figure 4. See page 184 in the book. Electrons have a property called spin angular momentum which can take on two different values of opposite sign.
How many electrons can be accomodated in an orbital. A How many electrons can fit in an f sublevel a set of degenerate f orbitals. For which n3 and l1 is the sub orbit which has 3 orbital.
1 Answer Junaid Mirza May 9 2018 261014 respectively Explanation. Only two eloctrons can fit in an orbital. The 3d 4d etc can each hold ten electrons because they each have five orbitals and each orbital can hold two electrons 5210.
P1221 1 6. Period 1 through Period 3 orbitals plotted as increasing principle quantum number versus relative energy. Each s orbital hold 2 electrons 1 pair.
Each orbital can occupy a maximum of 2 electrons. Each orbital can contain a maximum of two electrons. S0220 1 2.
EduRev NEET Question is disucussed on EduRev Study Group by 790 NEET Students. Students should confuse orbitals with the subshells which are different. How many electrons in sulphur Z 1 6 can have n l 3.
An s-orbital holds 2 electrons. Each atomic orbital can contain up to two electrons. The first orbit only has an s orbital.
ANSWER- number of electron fit in the orbital n3and l1 is 6Total. Thus to find the number of electrons possible per shell. Thus n1 shell can hold two electrons.
It turns out that electrons residing in the same atomic orbital must have opposite spin angular momenta. So in that particular sub orbit maximum 6 electrons can be fitted. Any p -orbital can accommodate up to.
And every orbital has maximum two electrons. How many orbitals are there in the third shell. The subshells which include s p d and f the contain the orbitals.
For any orbital it will always be 2 electrons But for Sub levels you multiply its orbitals x2 for example d 5 orbitals 5 x 2 10 electrons So a. The fourthenergy level has 18 electrons. First we look at the n1 shell the first shell.
Thus the thirdlevel holds a maximum of 18 electrons. Three orbitals How many electrons can the 4th orbital hold. B How many electrons can fit in one f orbital just one of the degenerate orbitals.
If ℓ is the angular quantum number of subshell then maximum electrons it can hold is 22ℓ 1 Sub-shellℓMaximum electrons. Two are in its innermost 1s orbital and eight fill its second shell two each in the 2s and three p orbitals. The five d orbitals can hold up to 10 electrons.
How Many Electrons Are In Each Shell Including 3p Orbitals
How Many Electrons Can Fit In The First Energy Level At Level

The First Image Ever Of A Hydrogen Atom S Orbital Structure Fun Science Wave Function Hydrogen Atom

How Many Electrons Can Fit Into The Orbitals That Comprise The 3 Rd Quantum Shell N 3 Youtube
0 Comments